26. Asande L.K, Ombori O., Nyaboga E.N., Oduor R.O. (2020), Efficient shoot organogenesis using leaf disc and nodal explant of passion fruit (Passiflora edulisSims.) and genetic fidelity assessment using Sequence-Related Amplified Polymorphism (SRAP) marker, International Journal of Agronomy, 2020, pp.1-10.
27. Attridge T.H. (1990), Light and plant reponses, Edward Arnold, London.
28. Banu S.G., Kumar G., Rajasekara P.M. (2007), Establishment of shoot cultures and plant regeneration of Passiflora edulis Sims., Indian Journal of Plant Physiology, 12(1), pp. 23-27.
29. Becerra D.C., Forero A.P., G´ongora G.A. (2004), Age and physiological condition of donor plants affect in vitro morphogenesis in leaf explants of Passiflora edulis f. flavicarpa, Plant Cell Tissue and Organ Culture, 79(1), pp. 87-90.
30. Bellamine J., Penel C., Greppin H., Gaspar T. (1998), Confirmation of the role of auxin and calcium in the late phases of adventitious root formation, Plant Growth Regulation, 26(3), pp. 191-194.
31. Bhatia P., Ashwath N. (2005), Effect of duration of light: Dark cycles on in vitro shoot regeneration of tomato, Asian Journal of Plant Sciences, 4(3), pp. 255-260.
32. Biasi L.A., Falco M.C., Rodriguez A.P.M, Mendes B.M.J. (2000), Organogenesis from internodal segments of yellow passion fruit, Scientia Agricola, 57(4), pp. 661-665.
33. Bressan P.H., Kim Y.J., Hyndman S.E., Hasegawa P.M., Bressan R.A. (1982), Factors affecting in vitro propagation of rose, Journal of the American Society for Horticultural Science, 107(6), pp. 979-990.
34. Brunt, A., Crabtree, K., Gibbs, A. (1990), Viruses of Tropical Plants, Commonwealth Agricultural Bureaux International, Wallingford, UK.
35. Chattopadhyaya B., Banerjee J., Basu A., Sen S.K., Maiti M.K. (2010), Shoot induction and regeneration using internodal transverse thin cell layer culture in Sesamum indicum L., Plant Biotechnology Reports, 4(2), pp. 173-178.
Có thể bạn quan tâm!
-
 Sơ Đồ Quy Trình Nhân Giống Chanh Dây Tím Và Vàng Thông Qua Kỹ Thuật Nuôi Cấy Tcl.
Sơ Đồ Quy Trình Nhân Giống Chanh Dây Tím Và Vàng Thông Qua Kỹ Thuật Nuôi Cấy Tcl. -
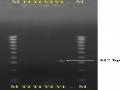
 Kết Quả Điện Di Trên Gel Agarose Của Các Mẫu Giống Chanh Dây Tím Và Vàng Đối Với Potyvirus.
Kết Quả Điện Di Trên Gel Agarose Của Các Mẫu Giống Chanh Dây Tím Và Vàng Đối Với Potyvirus. -
 Sơ Đồ Quy Trình Nhân Giống Chanh Dây Vi Ghép Sạch Virus Thông Qua Kỹ Thuật Nuôi Cấy Mô Phân Sinh Đỉnh, Nuôi Cấy Tcl Và Vi Ghép.
Sơ Đồ Quy Trình Nhân Giống Chanh Dây Vi Ghép Sạch Virus Thông Qua Kỹ Thuật Nuôi Cấy Mô Phân Sinh Đỉnh, Nuôi Cấy Tcl Và Vi Ghép. -
 Nghiên cứu nhân giống cây chanh dây Passiflora edulis bằng kỹ thuật nuôi cấy lớp mỏng tế bào và thử nghiệm tạo cây vi ghép - 21
Nghiên cứu nhân giống cây chanh dây Passiflora edulis bằng kỹ thuật nuôi cấy lớp mỏng tế bào và thử nghiệm tạo cây vi ghép - 21 -
 Kết Quả Phân Tích Thống Kê Khả Năng Khử Trùng Các Nguồn Mẫu Của Chanh Dây Tím Sau 4 Nuôi Cấy
Kết Quả Phân Tích Thống Kê Khả Năng Khử Trùng Các Nguồn Mẫu Của Chanh Dây Tím Sau 4 Nuôi Cấy -
 Kết Quả Phân Tích Thống Kê Ảnh Hưởng Của Chất Khử Trùng Và Nguồn Mẫu Lên Khả Năng Tái Sinh Chồi Giống Chanh Dây Tím Sau 8 Tuần Nuôi Cấy
Kết Quả Phân Tích Thống Kê Ảnh Hưởng Của Chất Khử Trùng Và Nguồn Mẫu Lên Khả Năng Tái Sinh Chồi Giống Chanh Dây Tím Sau 8 Tuần Nuôi Cấy
Xem toàn bộ 242 trang tài liệu này.
36. Chau N.H., Bang L., Buu N., Dung T., Ha H., Quang D. (2008), Some results in manufacturing of nano silver and investigation of its application for disinfection, Advances in Natural Sciences: Nanoscience and Nanotechnology, 9(2), pp. 241-248.
37. Chen Y.C, Chang C., Lin H.L. (2020), Topolins and red light improve the micropropagation efficiency of passion fruit (Passiflora edulis Sims.)

„Tainung No. 1‟, Hortscience, 55(8), pp. 1337-1344.
38. Choudhary M.L. (1991), Effect of medium components and explant position on in vitro shoot proliferation of Indian rose, National Academy Science Letters, 14(1), pp. 439-441.
39. Christensen B., Sriskandarajah S., Serek M., Muller R. (2008), In vitro culture of Hibiscus rosa-sinensis L.: Influence of iron, calcium and BAP on establishment and multiplication, Plant Cell, Tissue and Organ Culture, 93(2), pp. 151-161.
40. Croom L.A., Jackson C.L., Vaidya B.N., Parajuli P., Joshee N. (2016), Thin cell layer (TCL) culture system for herbal biomass production and genetic transformation of Bacopa monnieri L. Wettst., American Journal of Plant Sciences, 7(8), pp. 1232-1245.
41. Da Silva C.V., De Oliveira L.S., Loriato V.A.P., Da Silva L.C., De Campos J.M.S., Viccini L.F., De Oliveira E.J., Otoni W.C. (2011), Organogenesis from root explants of commercial populations of Passiflora edulis Sims. and a wild passion fruit species, P. cincinnata Masters, Plant Cell Tissue and Organ Culture, 107(3), pp. 407-416.
42. Davies P.J. (1995), The Plant Hormones: Their Nature, Occurrence, and Functions, In: Davies P.J. (Ed.), Plant Hormones: Physiology, Biochemistry, and Molecular Biology, Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 1-5.
43. Debergh P.C. (1983), Effects of agar brand and concentration on the tissue culture medium, Physiologia Plantarum, 59(2), pp. 270-276.
44. Dias L.L.C., Santa-Cataria C., Ribeiro DM.R., Barros R.S., Floh E.I.S, Otoni
W.C. (2009), Ethylene and polyamine production patterns during in vitro shoot organogenesis of two passion fruit species as affected by polyamines and their inhibitor, Plant Cell Tissue and Organ Culture, 99(2), pp. 199-208.
45. Dobránszki J., Teixeira da Silva J.A. (2010), Micropropagation of an apple a review, Biotechnology Advances, 28(4), pp. 462-488.
46. Dobránszki J., Teixeira da Silva J.A. (2011), Adventitious shoot regeneration from leaf thin cell layers in apple, Scientia Horticulturae, 127(3), pp. 460-463.
47. Dobránszki J., Teixeira da Silva J.A. (2013), In vitro shoot regeneration from transverse thin cell layers of apple leaves in response to various factors, Journal of Horticultural Science & Biotechnology, 88(1), pp. 60-66.
48. Dornelas M.C., Vieira M.L.C. (1994), Tissue culture studies on species of
Passiflora, Plant Cell, Tissue and Organ Culture, 36(2), pp. 211-217.
49. Duncan D.B. (1955), Multiple range and multiple F test, Biometrics, 11, pp. 1- 42.
50. Economou A.S., Read P.E. (1986), Influence of light duration and irradiance on micropropagation of a hardy deciduous azalea, Journal of the American Society for Horticultural Science, 111(1), pp. 146-149.
51. El-Sharabasy S.F., Ghazzawy H.S., Munir M. (2017), In vitro application of silver nanoparticles as explant disinfectant for date palm cultivar Barhee, Journal of Applied Horticulture, 19(2), pp. 106-112.
52. Fakhrfeshani M., Bagheri A., Sharifi A. (2012), Disinfecting effects of nano silver fluids in Gerbera (Gerbera jamesonii) capitulum tissue culture, Advances in Horticultural Science, 6(17), pp. 121-127.
53. Faria J.L.C., Segura J. (1997a), Micropropagation of yellow passion fruit by axillary bud proliferation, Hortscience, 32(7), pp. 1276-1277.
54. Faria J.L.C., Segura J. (1997b), In vitro control of adventitious bud differentiation by inorganic medium components and silver thiosulfate in explants of Passiflora edulis f. flavicarpa, In Vitro Cellular and Developmental Biology-Plant, 33(3), pp. 209-212.
55. Fernando J.A., Vieira M.L.C., Machado S.R., Appezzato-Da-Gloria B. (2007), New insights into the in vitro organogenesis process: the case of Passiflora, Plant Cell Tissue and Organ Culture, 91(1), pp. 37-44.
56. Figueira A., Janick J. (1994), Optimizing carbon dioxide and light levels during in vitro culture of Theobroma cacao, Journal of the American Society for Horticultural Science, 119(4), pp. 865-871.
57. Fischer I.H., Rezende J.A.M. (2008), Diseases of Passion flower (Passiflora spp.), Pest Technology, 2(1), pp. 1-19.
58. Fujiwara K., Kozai T., Watanabe I. (1987), Measurements of carbon dioxide gas concentration in closed vessels containing tissue cultured plantlets and estimates of net photosynthetic rates of the plantlets, Journal Agricultural and Forest Meteorology, 43(1), pp. 21-30.
59. Garcia R., Pacheco G., Falcão E., Borges G., Mansur E. (2011), Influence of type of explant, plant growth regulators, salt composition of basal medium, and light on callogenesis and regeneration in Passiflora suberosa L. (Passifloraceae), Plant Cell, Tissue and Organ Culture, 106(1), pp. 47-54.
60. Gattuso S., Severin C., Salinas A., Gattuso M., Giubileo G., Aguirre A., Busilacchi H. (2003), Micropropagation of Passiflora caerulea L. and histological studies of tissue regeneration, Journal of Tropical Medicinal Plant, 4(2), pp. 249-256.
61. George E.F., Hall M.A., De Klerk G.J. (2008), The Components of Plant Tissue Culture Media I: Macro- and Micro-Nutrients, In: George E.F., Hall M.A., De Klerk G.J. (Eds.), Plant Propagation by Tissue Culture 3rd Ed., Exegetic, Basingstone, UK, pp. 65-113.
62. Ghanbar T., Hosseini B., Jabbarzadeh Z., Farokhzad A., Sharafi A. (2016), High-frequency in vitro direct shoots regeneration from axillary nodal and shoot tip explants of clary sage (Salvia sclarea L.), Bulgarian Journal of Agricultural Science, 22(1), pp. 73-78.
63. Gharati S., Zarghami R., Amiri M. (2010), Evaluation of nano silver application for eliminate contamination and viability cultured segment node of
Persian Walnut in in vitro condition, The 5st Conference of New Idea in Agriculture, Islamic Azad University, Khorasgan, Iran, pp. 16-17.
64. Goh C.J., Lakshmanan P., Loh C.S. (1994), High frequency direct shoot bud regeneration from excised leaves of mangosteen (Garcinia mangostana L.), Plant Science, 101(2), pp. 173-180.
65. Gomez-Leyva J.F., Martinez-Acosta L.A., Lopez-Muraira I.G., Silos-Espino H., Ramirez-Cervantes F., Andrade-Gonzalez I. (2008), Multiple shoot regeneration of roselle (Hibiscus sabdariffa L.) from a shoot apex culture system, International Journal of Botany, 4(3), pp. 326-330.
66. Griesbach J.A. (1995), Detection of Tomato Ringspot Virus by Polymerase Chain Reaction, Plant Disease, 79(1), pp.1054-1056.
67. Grout B.W.W. (1999), Meristem-tip culture for propagation and virus elimination, Plant cell culture protocols, 111(1), pp. 15-125.
68. Gupta S.D., Agarwal A. (2017), Infuence of LED lighting on in vitro plant regeneration and associated cellular redox balance, In: Gupta S.D. (Ed.), Light Emitting Diodes for Agriculture, Springer, Singapore, pp. 273-303.
69. Hall R.M., Drew R.A., Higgins C.M., Dietzgen R.G. (2000), Efficient organogenesis of an Australian passion fruit hybrid (Passiflora edulis x Passiflora edulis f. flavicarpa) suitable for gene delivery, Australian Journal of Botany, 48(5), pp. 673-680.
70. Hartmann H.T., Kester D.E., Davies F.T., Geneve R.L. (2002), Plant Propagation, Principles and Practices, Prentice Hall, New Jersey.
71. Hassankhah A., Vahdati K., Lotfi M., Mirmasoumi M., Preece J., Assareh
M.H. (2014), Effects of ventilation and sucrose concentrations on the growth and plantlet anatomy of micropropagated persian walnut plants, International Journal of Horticultural Science and Technology, 1(2), pp. 111-120.
72. Hsia C., Korban S.S. (1996), Factors affecting in vitro establishment and shoot proliferation of rosa Hybrida L. and rosa Chinensis Minim, In vitro Cellular and Developmental Biology-Plant, 32(4), pp. 217-222.
73. Huh Y.S, Lee J.K., Nam S.Y. (2017), Effect of plant growth regulators and antioxidants on in vitro plant regeneration and callus induction from leaf explants of purple passion fruit (Passiflora edulis Sims.), Journal of Plant Biotechnology, 44(3), pp. 335-342.
74. Hussian G., Wani M.S., Mir M.A., Rather Z.A., Bhat K.M. (2014), Micrografting for fruit crop improvement, African Journal of Biotechnology, 13(25), pp. 2474-2483.
75. Ines M., Krunoslav D., Vesna T., Marija V., Ankica P., Zlatko C., Boris P., Zorica J. (2013), In vitro sterilization procedures for micropropagation of Oblaciska sour cherry, Journal of Agricultural Science, 58(2), pp. 117-126.
76. Isutsa D.K. (2004), Rapid micropropagation of passion fruit (Passiflora edulis
Sims.) varieties, Scientia Horticulturae, 99(3-4), pp. 395-400.
77. Izzo L.G., Arena C., De Micco V., Capozzi F., Aronne G. (2019), Light quality shapes morpho-functional traits and pigment content of green and red leaf cultivars of Atriplex hortensis, Scientia Horticulturae, 246(2), pp. 942- 950.
78. Jafari M., Daneshvar M.H., Lotfi A. (2017), In vitro shoot proliferation of Passiflora caerulea L. via cotyledonary node and shoot tip explants, Biotechnologia, 98(2), pp. 113-119.
79. Jeffree C.E., Yeoman M.M (1983), Development of intercellular connections between opposing cells in graft unions, New Phytologist, 93(4), pp. 491-509.
80. Jing G.F., Razak W.N.A.W.A.B., Rahman Z.A.B., Subramaniam S. (2014), The effect of thin cell layer system in Vanilla planifolia in vitro culture, Current Botany, 5(1), pp. 22-25.
81. Kantharajah A.S., Dodd, W.A. (1990), In vitro micropropagation of passiflora edulis (purple passion fruit), Annals of Botany, 65(3), pp. 337-339.
82. Kendrick R.E., Kronenberg G.H.M. (1994), Photomorphogenesis in plants, Kluwer Academic Publishers, Springer Netherlands.
83. Kim Y.S., Hahn E.J., Yeung E.C., Paek K.Y. (2003), Lateral root development and saponin accumulation as affected by IBA or NAA in adventitious root
cultures of Panax ginseng C.A. Meyer, In Vitro Cellular and Developmental Biology-Plant, 39(2), pp. 245-249.
84. Kishore K., Pathak K.A., Yadav D.S, Bujarbaruah K.M., Bharali R., Shukla R. (2006), Passion fruit, ICAR research Complex for NEH region, Umiam- 793103, Meghalaya.
85. Kollmeier M., Mafelle H.H., Horst W.J. (2000), Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum?, Plant Physiology, 122(3), pp. 945-956.
86. Komathi S., Rajalakshmi G., Savetha S., Ayyappadas M.P. (2011), In vitro regeneration of Passiflora foetida L., Journal of Research in Biology, 8(1), pp. 653-659.
87. Kotsias D., Roussos P.A. (2001), An investigation on the effect of different plant growth regulating compounds in in vitro shoot tip and node culture of lemon seedlings, Scientia Horticulturae, 89(2), pp. 115-128.
88. Kozai T. (1991), Micropropagation under photoautotropic conditions, In: Debergh P.C., Zimmerman R.H. (Eds.), Micropropagation, Technology and Application, Kluwer Academic Publishers, Dordrecht, the Netherlands, pp. 447-469.
89. Kozai T., Sekimoto K. (1988), Effects of the number of air exchanges per hour of the closed vessel and the photosynthetic photon flux on the carbon dioxide concentration inside the vessel and the growth of strawberry plantlets in vitro, Environmental Control in Biology, 26(1), pp. 21-29.
90. Kumar S., Khan M.S., Raj S.K., Sharma A.K (2009), Elimination of mixed infection of Cucumber mosaic and Tomato aspermy virus from Chrysanthemum morifolium Ramat. cv. Pooja by shoot meristem culture, Scientia Horticulturae, 119(2), pp. 108-112.
91. Kumar V., Ramakrishna A., Ravishankar G.A. (2007), Influence of different ethylene inhibitors on somatic embryogenesis and secondary embryogenesis
from Coffea canephora P ex Fr., In Vitro Cellular and Developmental Biology-Plant, 43(6), pp. 602-607.
92. Kurilčik A., Miklušytė-Čanova R., Dapkūnienė S., Žilinskaitė S., Kurilčik G., Tamulaitis G., Duchovskis P., Žukauskas A. (2008), In vitro culture of Chrysanthemum plantlets using light-emitting diodes, Central European Journal of Biology, 3(2), pp. 161-167.
93. Le B.V., Ha N.T., Hong L.T.A, Tran Thanh Van K. (1999), High frequency shoot regeneration from trifoliate orange (Poncirus trifoliata L. Raf.) using the thin cell layer method, Life Sciences, 322(12), pp. 1105-1111.
94. Lee-Stadlemann O.Y., Lee S., Hackett W.P., Read P.E. (1989), The formation of adventitious buds in vitro on micro-cross sections of hybird Populus leaf midveins, Plant Science, 61(2), pp. 263-272.
95. Lercari B. Manetti A., Bertram L. (2002), Temporal and spatial pattern of light-dependent acquisition of competence for shoot formation in tomato hypocotyl: Light pulse conditions, Advances in Horticultural Science, 16(1), pp. 17-24.
96. Li H., Xu Z., Tang C. (2010), Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro, Plant Cell, Tissue and Organ culture, 103(2), pp. 155-163.
97. Lim K.B., Kwon S.J., Lee S.I., Hwang Y.J., Naing A.H. (2012), Influence of genotype, explant source, and gelling agent on in vitro shoot regeneration of Chrysanthemum, Horticulture, Environment and Biotechnology, 53(4), pp. 329-335.
98. Lombardi S.P., Passos I.S., Nogueira M.C.S., da Glória B.A. (2007), In vitro shoot regeneration from roots and leaf discs of Passiflora cincinnata Mast., Brazilian Archives of Biology and Technology, 50(2), pp. 239-247.
99. Ludford P.M. (1995), Postharvest hormone changes in vegetables and fruit, In: Davies P.J. (Ed.), Plant hormones, Dordrecht: Kluwer Academic Publishers, pp. 725-750.