172. Rahul Kumar, Neeraj Goel, Ramesh Raliya, Pratim Biswas and Mahesh Kumar (2018), "High-performance photodetector based on hybrid of MoS2 and reduced graphene oxide ", Nanotechnology. 29(40), tr. 1-17.
173. Thillai Sivakumar Natarajan, Kalithasan Natarajan, Hari C. Bajaj, Rajesh J. Tayade (2013), "Enhanced photocatalytic activity of bismuth-doped TiO2 nanotubes under direct sunlight irradiation for degradation of Rhodamine B dye ", J Nanopart Res 15(15), tr. 1-18.
174. Xiufeng Zhou, Juan Lu, Jingjing Jiang, Xiaobin Li, Mengna Lu, Guotao Yuan, Zuoshan Wang, Min Zheng and Hyo Jin Seo (2014), "Simple fabrication of N-doped mesoporous TiO2 nanorods with the enhanced visible light photocatalytic activity ", Nanoscale Research Letters 9(34), tr. 1-7.
Construction of MoS2-g-C3N4 Heterostructures Using Mechanochemistry for High Performance Electrochemical Supercapacitor and Visible Light Photocatalytic Applications", Nature Scientific Report. 7, tr. 43055-43066.
176. Xiaoru Guo, Yang Hou, Ren Ren and Junhong Chen (2017), "Temperature- dependent Crystallization of MoS2 Nanoflakes on Graphene Nanosheets for Electrocatalysis ", Nanoscale Research Letters 12(479), tr. 1-9.
177. Honglin Li, KeYu, Chao Li, ZhengTang, BangjunGuo, Xiang Lei, Hao Fu & Ziqiang Zhu (2015), "Charge-Transfer Induced High Efficient Hydrogen Evolution of MoS2/graphene Cocatalyst ", Scientific Reports 5(1), tr. 1-11.
178. G.U. Ryu, G.M. Kim, Hammad R. Khalid and H.K. Lee (2019), "The Effects of Temperature on the Hydrothermal Synthesis of Hydroxyapatite-Zeolite Using Blast Furnace Slag", Materials 12, tr. 2131-2143.
Heterointerface in MoS2/Reduced Graphene Oxide Composites," RSC Adv., .
180. S. Kumar, V. Sharma, K. Bhattacharyya and V. Krishnan (2016), "Synergetic effect of MoS2-RGO doping to enhance the photocatalytic performance of ZnO nanoparticles", New J. Chem. . 40, tr. 5185-5197.
181. M. K. Singh, P. Chettri, A. Tripathi, A. Tiwari, B. Mukherjee and R. K. mandal (2018), "Defect mediated magnetic transitions in Fe and Mn doped MoS2 ", Phys. Chem. Chem. Phys. . 20, tr. 15817-15823.
Có thể bạn quan tâm!
-
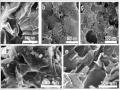
 Ảnh Sem Của Các Mẫu Vật Liệu Rgo (A), Mos 2 (B), 3%mn-Mos 2 (C), 3%mn- Mos 2 /rgo (D) Và Mos 2 /rgo (E)
Ảnh Sem Của Các Mẫu Vật Liệu Rgo (A), Mos 2 (B), 3%mn-Mos 2 (C), 3%mn- Mos 2 /rgo (D) Và Mos 2 /rgo (E) -
 Phan Thi Thuy Trang , Truong Cong Duc, Truong Thanh Tam, Vo Vien, Nguyen Hong Lien, “Effect Of Mn 2+ Dopants On The Photocatalytic Efficiency Of Mos 2 ” , Vietnam Journal Of Catalysis And
Phan Thi Thuy Trang , Truong Cong Duc, Truong Thanh Tam, Vo Vien, Nguyen Hong Lien, “Effect Of Mn 2+ Dopants On The Photocatalytic Efficiency Of Mos 2 ” , Vietnam Journal Of Catalysis And -
 Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 19
Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 19 -
 Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 21
Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 21 -
 Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 22
Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 22 -
 Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 23
Nghiên cứu tổng hợp, đặc trưng xúc tác MoS2/rGO biến tính với Mn và ứng dụng cho quá trình quang phân hủy rhodamine B trong vùng ánh sáng khả kiến - 23
Xem toàn bộ 192 trang tài liệu này.
182. Jieqiong Wang, Fan Sun, Sen Yang, Yitong Li, Chuan Zhao, Minwei Xu, Yin Zhang, and Hao Zeng (2016), "Robust ferromagnetism in Mn-doped MoS2 nanostructures", Applied Physics Letter 109, tr. 1-6.
183. Shannon, R. D. (1976), "Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides", Acta Cryst. A32, tr. 751.

184. Neeru Sharma, Vikas Sharma, Yachana Jain, Mitlesh Kumari, Ragini Gupta,
S. K. Sharma, and K. Sachdev (2017), "Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application", Macromolecular Symposia,. 376(1), tr. 1-5.
185. H., Guo (2009), "A green approach to the synthesis of graphenenanosheets",
ACS Nano 3, tr. 2653–2659.
186. Zhang TY, Zhang D. (2011), "Aqueous colloids of graphene oxide nanosheets by exfoliation of graphite oxide without ultrasonication", Bull Mater. Sci. . 451(34), tr. 25-28.
187. Bin Chen, Bao-Jia Ni, Meng-Xiang Fu, Hang Zhong, Wei-Feng Jiang, Si- Yuan Liu, He-Xin Zhang, and Keun-Byoung Yoon (2019), "Effect of Molybdenum Disulfide Exfoliation Conditions on the Mechanical Properties of Epoxy Nanocomposites ", Chinese J. Polym. Sci. . 37, tr. 687–692.
188. Gao D, Zhang J, Yang G, Qi J, Si M, Xue D (2011), "Ferromagnetism induced by oxygen vacancies in zinc peroxide nanoparticles", J. Phys. Chem. C. . 115, tr. 16405–16410.
189. Ma YW, Lu YH, Yi JB, Feng YP, Herng TS, Liu X, Gao DQ, Xue DS, Xue JM, Ouyang JY, Ding J (2012), "Room temperature ferromagnetism in Teflon due to carbon dangling bonds", Nat. Commun. . 3, tr. 727-735.
190. Vernekar, Beena K (2019), "ESR Spectral Characterisation of Mn doped Zn
(II) Trisethylenediamine Sulfidometalate Complexes ", International Journal of Innovative Technology and Exploring Engineering (IJITEE) 9(2S3), tr. 2278-3075.
191. Babic-Stojic B, Milivojevic D, Blanusa J, Spasojevic V, Bibic N, Simonovic B, Arandelovic D (2008), "Ferromagnetic properties of the Zn-Mn-O system", J. Phys. Condens. Matter. 20(23), tr. 1-8.
192. Xi Luo, Wai-Tung Lee, Guozhong Xing, Nina Bao, Adnan Yonis, Dewei Chu, Jiunn Lee, Jun Ding, Sean Liand Jiabao Yi (2014), "Ferromagnetic ordering in Mn-doped ZnO Nanoparticles", Nanoscale Research Letters 9(625), tr. 1-8.
193. Tseng, L.-T., Luo, X., Tan, T.T., Li, S., Yi, J. (2014), "Doping concentration dependence of microstructure and magnetic behaviours in Co-doped TiO2 nanorods", Nanoscale Res. Lett. 9(1), tr. 1-10.
194. Mianzeng Zhong, Chao Shen, Le Huang, Hui-Xiong Deng, Guozhen Shen, Houzhi Zheng, Zhongming Wei and Jingbo Li (2019), "Electronic structure and exciton shifts in Sb-doped MoS2 monolayer", Materials and Applications 3(1), tr. 1-7.
195. Kui Chen, Mei Wang, Guangli Li, Quanguo He, Jun Liu and Fuzhi Li (2018), "Spherical -MnO2 Supported on N-KB as Efficient Electrocatalyst for Oxygen Reduction in Al–Air Battery ", Materials 11(4), tr. 601-615.
Reduction of NOx with Ammonia over Mn-Modified Fe2O3/AC Catalysts", J. Braz. Chem. Soc. . 29(1), tr. 79-87.
197. VishwanathHiremath, Min Cho and Jeong Gil Seo (2018), "Self-assembled Mn3O4 nano-clusters over carbon nanotube threads with enhanced supercapacitor performance ", New J. Chem. . 42, tr. 19608-19614.
198. Yuan Ma, Yanjiao Ma, Guk-Tae Kim, Thomas Diemant, Rolf Jürgen Behm, Dorin Geiger, Ute Kaiser, Alberto Varzi, and Stefano Passerini (2019), "Superior Lithium Storage Capacity of α-MnS Nanoparticles Embedded in S-Doped Carbonaceous Mesoporous Frameworks", Adv. Energy Mater. 1902077, tr. 1-15.
199. A. Syari'ati, S.Kumar, A. Zahid, A. A. El Yumin, J. Ye and P. Rudolf (2019), "Photoemission Spectroscopy Study of Structural Defects in Molybdenum disulfide (MoS2) Grown by Chemical Vapor Deposition (CVD)", Chem. Commun. 55, tr. 10384-10387.
200. A. Wang, H. Wang, S. Zhang, C. Mao, J. Song, H. Niu, B. Jin, Y. Tian (2013), "Controlled synthesis of nickel sulfide/graphene oxide nanocomposite for high-performance supercapacitor ", Appl. Surf. Sci. . 282, tr. 704–708.
201. Y. Yang, Y. Li, L. Zhu, H. He, L. Hu, J.Huang, F. Hu, B. He and Z. Ye (2013), "Shape control of colloidal Mn doped ZnO nanocrystals and their visible light photocatalytic properties ", Nanoscale Res. Lett. 5, tr. 10461- 10471.
202. F.A. Aisien, N.A. Amenaghawon, and E.F. Ekpenisi (2013), "Photocatalytic decolourisation of industrial wastewater from a soft drink company", Journal of Engineering and Applied Sciences. 9, tr. 11-16.
203. M. A. Behnajady, N. Modirshahla, R. Hamzavi (2006), "Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst", J. Hazard.Mater.,. B133, tr. 226–232.
204. Kumar A, Pandey G. (2017), "A review on the factors affecting the photocatalytic degradation of hazardous materials", Material Science & Engineering International Journal. 1(3), tr. 106-114.
205. Alsaady, Hazim Y. Al-gubury and Hedear H. (2015), "Photocatalytic Degradation of Rhodamine B using Titanium Dioxide", International Journal of Multidisciplinary and Current Research. 3, tr. 1-7.
206. A. Akbar Isari, A. Payan, M. Fattahi, S. Jorfi, B. Kakavandi (2018), "Photocatalytic degradation of Rhodamine B and Real Textile Wastewater using Fe-Doped TiO2 anchored on Reduced Graphene Oxide (FeTiO2/rGO): Characterization and feasibility, mechanism and pathway studies", Applied Surface Science. 462, tr. 549-564.
207. H.Y.He, J.F.Huang, L.Y.Cao, and J.P.Wu (2010), "Photodegradation of methyl orange aqueous on MnWO4 powder under different light resources and initial pH", Desalination. 252, tr. 66-70.
208. N. Venkatachalam, M. Palanichamy, B. Arabindoo, and V. Murugesan (2007), "Enhanced photocatalytic degradation of 4-chlorophenol by Zr4+ doped nano TiO2", Journal of Molecular Catalysis A: Chemical. 266, tr. 158–165.
209. Pei Pei Gan, Sam Fong Yau Li (2013), "Efficient removal of Rhodamine B using a rice hull-based silica supported iron catalyst by Fenton-like process", Chemical Engineering Journal. 259, tr. 351-363.
210. Meriem Zamouche, Oualid Hamdaoui (2012), "Sorption of Rhodamine B by cedar cone: effect of pH and ionic strength", Energy Procedia. 18, tr. 1228- 1239.
211. Lin, Y.-J. Chiang and C.-C. (2013), "Photocatalytic decolorization of methylene blue in aqueous solutions using coupled ZnO/SnO2 photocatalysts ", Powder Technology. 246, tr. 137–143.
212. S. Rajoriya, S. Bargole, V. K. Saharan (2017), "Degradation of a cationic dye (Rhodamine 6G) using hydrodynamic cavitation coupled with other oxidative agents: Reaction mechanism and pathway", Ultrason. Sonochem. 34, tr. 183-194.
213. K. Jothivenkatachalam, S. Prabhu, A. Nithyaa and K. Jeganathan (2014), "Facile synthesis of WO3 with reduced particle size on zeolite and enhanced photocatalytic activity", RSC Adv. 4, tr. 21221-21229.
214. Min Zhang, Hong-fei Yin, Jia-cheng Yao, Muhammad Arif, Bo Qiu, Peng- fei Li, Xiao-heng Liu (2020), "All-solid-state Z-scheme BiOX(Cl, Br)-Au- CdS heterostructure: Photocatalytic activity and degradation pathway", Colloids and Surfaces A. 602, tr. 124778-124789.
215. Wei Li, Yongli Zhang, Pingju Zhao, Peng Zhou, Yang Liu, Xin Cheng, Jingquan Wang, Bo Yang, Hongguang Guo (2020), "Enhanced kinetic performance of peroxymonosulfate/ZVI system with the addition of copper ions: Reactivity, mechanism, and degradation pathways", Journal of Hazardous Materials. 393, tr. 122399-122436.
216. Yuting Zhang, Zhurui Shen, Zekun Xin, Zhuofeng Hu, Huiming Ji (2019), "Interfacial charge dominating major active species and degradation pathways: An example of carbon based photocatalyst", J Colloid Interface Sci. 554, tr. 743-751.
217. Doan An Tran, Chi Thanh Nguyen Pham, Tri Nguyen Ngoc, Hung Nguyen Phi,, Qui Thanh Hoai Ta, Duy Huong Truong, Van Thang Nguyen, Huy Hoang Luc, và Le Tuan Nguyen, Ngoc Nhiem Dao, Sung Jin Kim, Vien Vo (2020), "One-step synthesis of oxygen doped g-C3N4 for enhanced visible- light photodegradation of Rhodamine B", Journal of Physics and Chemistry of Solids. 151, tr. 109900-109911.
218. Lin H, Xu Z, Zhang L, Zhang Z, Xue L. (2019), "The effects of different surfactants on the morphologies and electrochemical properties of MoS2/reduce graphene oxide composites", Chem. Phys. Lett. . 716, tr. 6-10.
219. Shixiong Min, and Gongxuan Lu. (2012), "Sites for High Efficient Photocatalytic Hydrogen Evolution on a Limited-Layered MoS2 Cocatalyst Confined on Graphene Sheets-The Role of Graphene", J. Phys. Chem. C 116, tr. 25415-25424.
220. Leqiang Shao, Deli Jiang, Peng Xiao, Liming Zhu, Suci Meng, Min Chen (2016), "Enhancement of g-C3N4 nanosheets photocatalysis by synergistic interaction of ZnS microsphere and RGO inducing multistep charge transfer", Applied Catalysis B: Environmental. 198(5), tr. 200-210.
221. Hongfei Pan, Xiaona Zhao, Zhanming Fu, Wenmao Tu, Pengfei Fang, Haining Zhang (2018), "Visible-light induced photocatalysis of AgCl@Ag/titanate nanotubes/nitrogen-doped reduced graphite oxide composites", Applied Surface Science. 442, tr. 547-555.
PHỤ LỤC
Phụ lục 1. Giản đồ XRD của các mẫu vật liệu graphit
8000
Faculty of Chemistry, HUS, VNU, D8 ADVANCE-Bruker - Graphite
7000
d=3.368
6000
5000
Lin (Cps)
4000
3000
2000
d=2.037
d=1.682
1000
0
10 20 30 40 50 60 70 8
2-Theta - Scale
File: TrangQNU Graphite.raw - Type: 2Th/Th locked - Start: 10.000 ° - End: 79.990 ° - Step: 0.030 ° - Step time: 0.3 s - Temp.: 25 °C (Room) - Time Started: 14 s - 2-Theta: 10.000 ° - Theta: 5.000 ° - Chi: 0.00 ° - Phi: 0.00 ° - 00-041-1487 (I) - Graphite-2H - C - Y: 47.39 % - d x by: 1. - WL: 1.5406 - Hexagonal - a 2.47040 - b 2.47040 - c 6.72440 - alpha 90.000 - beta 90.000 - gamma 120.000 - Primitive - P63/mmc (194) - 4 - 35.5401 - I/Ic PDF 7.
Phụ lục 2. Giản đồ XRD của các mẫu vật liệu GO
Faculty of Chemistry, HUS, VNU, D8 ADVANCE-Bruker - GO
400
d=7.647
300
Lin (Cps)
200
d=2.142
100
0
2 10 20 30 40 50 60 70 8
2-Theta - Scale
File: ThaoQNU GO.raw - Type: 2Th/Th locked - Start: 2.000 ° - End: 80.000 ° - Step: 0.030 ° - Step time: 0.5 s - Temp.: 25 °C (Room) - Time Started: 23 s - 2-Theta: 2.000 ° - Theta: 1.000 ° - Chi: 0.00 ° - Phi: 0.00 ° - X: 0.0
Phụ lục 3. Giản đồ XRD của các mẫu vật liệu rGO ở các nhiệt độ nung khác nhau
3.1. rGO ở 70oC
d=3.371
d=2.944
d=1.84
d=1.670
d=1.363d=1.313
Lin (Cps)
Faculty of Chemistry, HUS, VNU, D8 ADVANCE-Bruker - rGO 70
![]()
2-Theta - Scale
File: TrangQNU RGO.raw - Type: 2Th/Th locked - Start: 2.000 ° - End: 80.000 ° - Step: 0.030 ° - Step time: 0.5 s - Temp.: 25 °C (Room) - Time Started: 25 s - 2-Theta: 2.000 ° - Theta: 1.000 ° - Chi: 0.00 ° -
3.2. rGO ở 200oC
1000
Faculty of Chemistry, HUS, VNU, D8 ADVANCE-Bruker - rGO 200
900
800
700
600
Lin (Cps)
500
d=3.591
400
300
200
100
0
2 10 20 30 40 50 60 70 8
2-Theta - Scale
File: TrangQNU rGO200.raw - Type: 2Th/Th locked - Start: 2.000 ° - End: 80.000 ° - Step: 0.030 ° - Step time: 0.5 s - Temp.: 25 °C (Room) - Time Started: 28 s - 2-Theta: 2.000 ° - Theta: 1.000 ° - Chi: 0.00 ° - Phi: 0.00 ° - X:
3.3. rGO ở 400oC
Faculty of Chemistry, HUS, VNU, D8 ADVANCE-Bruker - rGO 400
600
500
400
Lin (Cps)
d=3.470
300
200
100
0
2 10 20 30 40 50 60 70 8
2-Theta - Scale
![]() File: TrangQNU rGO400.raw - Type: 2Th/Th locked - Start: 2.000 ° - End: 80.000 ° - Step: 0.030 ° - Step time: 0.5 s - Temp.: 25 °C (Room) - Time Started: 15 s - 2-Theta: 2.000 ° - Theta: 1.000 ° - Chi: 0.00 ° - Phi: 0.00 ° - X:
File: TrangQNU rGO400.raw - Type: 2Th/Th locked - Start: 2.000 ° - End: 80.000 ° - Step: 0.030 ° - Step time: 0.5 s - Temp.: 25 °C (Room) - Time Started: 15 s - 2-Theta: 2.000 ° - Theta: 1.000 ° - Chi: 0.00 ° - Phi: 0.00 ° - X:
3.4. rGO ở 600oC
Faculty of Chemistry, HUS, VNU, D8 ADVANCE-Bruker - rGO 600
400
300
Lin (Cps)
d=3.419
200
100
0
2 10 20 30 40 50 60 70 8
2-Theta - Scale
File: TrangQNU RGOapril.raw - Type: 2Th/Th locked - Start: 2.000 ° - End: 80.000 ° - Step: 0.030 ° - Step time: 0.5 s - Temp.: 25 °C (Room) - Time Started: 15 s - 2-Theta: 2.000 ° - Theta: 1.000 ° - Chi: 0.00 ° - Phi: 0.00 ° -
1) Left Angle: 22.430 ° - Right Angle: 28.940 ° - Left Int.: 19.7 Cps - Right Int.: 29.8 Cps - Obs. Max: 25.970 ° - d (Obs. Max): 3.428 - Max Int.: 166 Cps - Net Height: 141 Cps - FWHM: 1.577 ° - Chord Mid.: 25.886 ° - Int. Br