164. Zi Y, Zhang B, Jiang B, Yang X, Liang Z, Liu W, He C, Liu L(2018), “Antioxidant action and protective and reparative effects of lentinan on oxidative damage in HaCaT cells”, Journal of Cosmetic Dermatology, 17(6), pp. 1108-1114.
165. Ren G, Xu L, Lu T, Zhang Y, Wang Y, Yin J(2019), “Protective effects of lentinan on lipopolysaccharide induced inflammatory response in intestine of juvenile taimen (Hucho taimen, Pallas)”, International Journal of Biological Macromolecules, 121, pp. 317-325.
166. Jang-Woo Shin, In-Chan Seol, Chang-Gue Son (2010), “Interpretation of Animal Dose and Human Equivalent Dose for Drug Development”, The Journal of Korean Oriental Medicine, 31(3), pp. 1-7.
167. Đỗ Trung Đàm (2014), Phương pháp xác định độc tính của thuốc, Nhà xuất bản y học, tr. 6-12.
168. Arunachalam, K., Sasidharan, S.P. (2021), Preclinical Drug Dose Calculation. In: Bioassays in Experimental and Preclinical Pharmacology, Springer Protocols Handbooks, Humana, New York, pp. 29-32.
169. Zhang, J., Zhou, B., Jin, S., Huang, Z., Ma, B., Shao, Q., & Zhu, W. (2020), “Mechanism of action of Panax notoginoside against lung cancer in mice based on response to CTSB gene”, BMC complementary medicine and therapies, 20(1), pp. 367.
170. Liu, Y. H., Qin, H. Y., Zhong, Y. Y., Li, S., Wang, H. J., Wang, H., Chen, L. L., Tang, X., Li, Y. L., Qian, Z. Y., Li, H. Y., Zhang, L., & Chen, T. (2021), “Neutral polysaccharide from Panax notoginseng enhanced cyclophosphamide antitumor efficacy in hepatoma H22-bearing mice”, BMC cancer, 21(1), pp. 37.
171. Zhao H, Han Z, Li G, Zhang S, Luo Y (2017), “Therapeutic Potential and Cellular Mechanisms of Panax notoginseng on Prevention of Aging and Cell Senescence-Associated Diseases”, Aging Dis, 8(6), pp. 721-739.
172. Gu CZ, Qiao YJ, Wang D, Zhu HT, Yang CR, Xu M, Zhang YJ (2018), “New triterpenoid saponins from the steaming treated roots of Panax notoginseng”, Nat Prod Res, 32(3), pp. 294-301.
173. He F, Ding Y, Liang C, Song S B, Dou DQ. Song GY, Kim YH (2014), “Antitumor effects of dammarane-type saponins from steamed Notoginseng”, Pharmacogn Mag, 10(39), pp. 314-317.
Có thể bạn quan tâm!
-
 Đỗ Tất Lợi (2019), Những Cây Thuốc Và Vị Thuốc Việt Nam (Tái Bản Lần Thứ 20),
Đỗ Tất Lợi (2019), Những Cây Thuốc Và Vị Thuốc Việt Nam (Tái Bản Lần Thứ 20), -
 Wei, G., Dong, L., Yang, J., Zhang, L., Xu, J., Yang, F., Cheng, R., Xu, R., & Chen, S. (2018), “Integrated Metabolomic And Transcriptomic Analyses Revealed The
Wei, G., Dong, L., Yang, J., Zhang, L., Xu, J., Yang, F., Cheng, R., Xu, R., & Chen, S. (2018), “Integrated Metabolomic And Transcriptomic Analyses Revealed The -
 Wilkins, R. C., Kutzner, B. C., Truong, M., Sanchez-Dardon, J., & Mclean, J.
Wilkins, R. C., Kutzner, B. C., Truong, M., Sanchez-Dardon, J., & Mclean, J. -
 Nghiên cứu tác dụng kháng u thực nghiệm của rễ củ Tam thất Panax notoginseng Burk. F.H. Chen, Araliaceae trồng ở Việt Nam trước và sau chế biến - 27
Nghiên cứu tác dụng kháng u thực nghiệm của rễ củ Tam thất Panax notoginseng Burk. F.H. Chen, Araliaceae trồng ở Việt Nam trước và sau chế biến - 27
Xem toàn bộ 222 trang tài liệu này.
174. XuC, LiuT, LiuH, ChenG, GuoY (2019), “Panax notoginseng saponins radiosensitize colorectal cancer cells by regulating the SNHG6/miR-137 axis”, RSC Adv, 9(6), pp. 38558-38567.
175. Zhang C, Tong X, Qi B, Yu X, Dong S, Zhang S, Li X, Yu M (2013), “Components of Panax notoginseng saponins enhance the cytotoxicity of cisplatin via their effects on gap junctions”, Mol Med Rep, 8(3), pp.897-902.
176. Wang CZ, Xie JT, Zhang B, et al (2007), “Chemopreventive effects of Panax notoginseng and its major constituents on SW480 human colorectal cancer cells”, Int J Oncol, 31(5), pp.1149-1156.
177. Sun M, Bu R, Zhang B, Cao Y, Liu C, Zhao W (2020), “Lentinan Inhibits Tumor Progression by Immunomodulation in a Mouse Model of Bladder Cancer”, Integrative cancer therapies, 19, pp. 1-7.
178. Berraondo P, Sanmamed MF, Ochoa MC et al (2019), “Cytokines in clinical cancer immunotherapy”, Br J Cancer, 120, pp. 6–15.
179. Jiang T, Zhou C, Ren S (2016), “Role of IL-2 in cancer immunotherapy”,
Oncoimmunology, 5(6), pp. e1163462.
180. Vannucci L, Krizan J, Sima P et al (2013), “Immunostimulatory properties and antitumor activities of glucans (Review)”, International Journal of Oncology, 43, pp. 357-364.
181. Dai J, Chen J, Qi J et al (2020), “Konjac Glucomannan from Amorphophallus konjac enhances immunocompetence of the cyclophosphamide-induced immunosuppressed mice”, Food Sci Nutr, 9(2), pp. 728-735.
182. Srinivas, U. S., Tan, B., Vellayappan, B. A., & Jeyasekharan, A. D. (2019), “ROS and the DNA damage response in cancer”, Redox biology, 25, pp. 101084.
183. Aboelella, N. S., Brandle, C., Kim, T., Ding, Z. C., & Zhou, G. (2021), “Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy”, Cancers, 13(5), pp. 986.
184. Muriel, P. and Gordillo, K.R., (2016), “Role of oxidative stress in liver health and disease”, Oxidative Medicine and Cellular Longevity, 2016, pp. 9037051.
185. Melekh, B., Ilkiv, I., Lozynskyi, A. and Sklyarov, A., (2017), “Antioxidant enzyme activity and lipid peroxidation in rat liver exposed to celecoxib and lansoprazole under epinephrine-induced stress”, Journal of Applied Pharmaceutical Science, 7, pp. 94-99.
186. Chen, T., Li, B., Qiu, Y., Qiu, Z., & Qu, P. (2018), “Functional mechanism of Ginsenosides on tumor growth and metastasis”, Saudi journal of biological sciences, 25(5), pp. 917–922.
187. Li W, Wang JQ, Zhou YD, Hou JG, Liu Y, Wang YP, Gong XJ, Lin XH, Jiang S, Wang Z, (2020), “Rare Ginsenoside 20(R)-Rg3 Inhibits D-Galactose- Induced Liver and Kidney Injury by Regulating Oxidative Stress-Induced Apoptosis”, The American Journal of Chinese Medicine, 48(5), pp. 1141-1157.
188. Hu, S., Zhu, Y., Xia, X., Xu, X., Chen, F., Miao, X., & Chen, X. (2019), “Ginsenoside Rg3 Prolongs Survival of the Orthotopic Hepatocellular Carcinoma Model by Inducing Apoptosis and Inhibiting Angiogenesis”, Analytical cellular pathology (Amsterdam), 2019, pp. 3815786.
189. Jiang, J. W., Chen, X. M., Chen, X. H., & Zheng, S. S. (2011), “Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via intrinsic apoptotic pathway”, World journal of gastroenterology, 17(31), pp. 3605–3613.
190. Aydιn, A., Aktay, G., & Yesilada, E. (2016), “A Guidance Manual for the Toxicity Assessment of Traditional Herbal Medicines”, Natural product communications, 11(11), pp. 1763–1773.
191. OECD (2002), Test No. 423: Acute Oral toxicity - Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris.
192. Erhirhie, E. O., Ihekwereme, C. P., & Ilodigwe, E. E. (2018), “Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance”, Interdisciplinary toxicology, 11(1), pp. 5–12.
193. OECD (2018), Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris.
194. Phạm Thị Minh Đức (2011), Sinh lý học (sách đào tạo bác sĩ đa khoa), Nhà xuất bản Y học, Hà Nội.
195. Đại học Y Hà Nội (2013), Hóa sinh lâm sàng, Nhà xuất bản Y học, Hà Nội.
196. Li, W., Zhang, X., Xin, Y., Xuan, Y., Liu, J., Li, P., & Zhao, Y. (2016), “Oral subchronic toxicity evaluation of a novel antitumor agent 25-methoxydammarane- 3, 12, 20-triol from Panax notoginseng in Sprague-Dawley rats”, Regulatory toxicology and pharmacology : RTP, 77, pp. 240–251.
197. Murbach, T. S., Glávits, R., Endres, J. R., Hirka, G., Vértesi, A., Béres, E., & Szakonyiné, I. P. (2019), “Toxicological Evaluation of a Mixture of Astragalus membranaceus and Panax notoginseng Root Extracts (InnoSlim®)”, Journal of toxicology, 2019, pp. 5723851.
PHỤ LỤC
Phụ lục 1: Phiếu giám định tên khoa học của dược liệu Tam thất


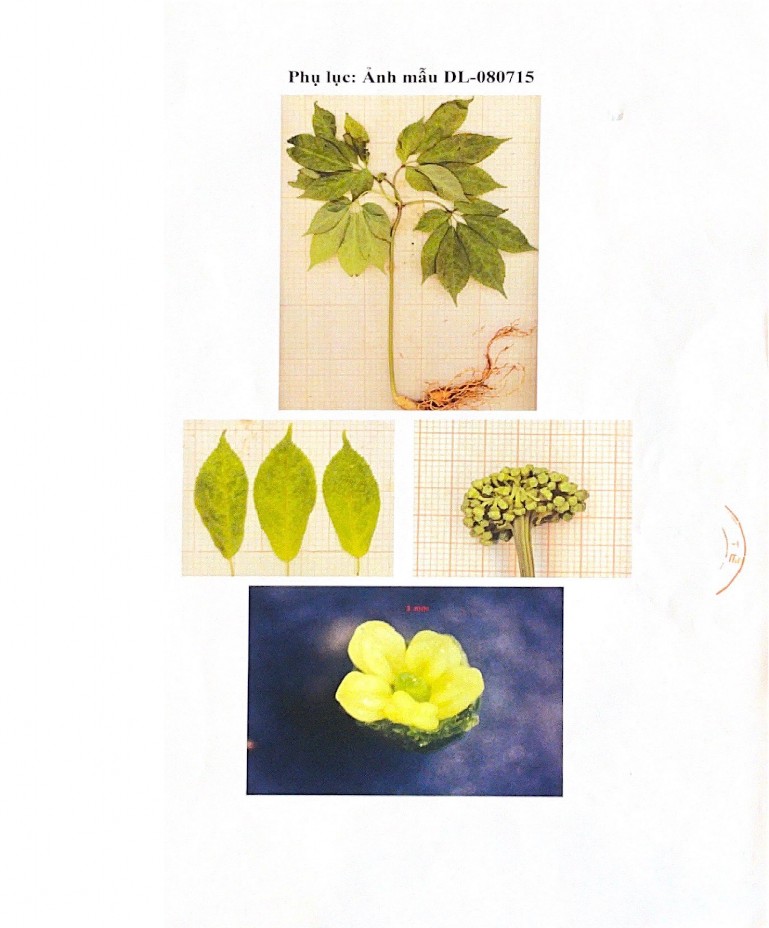
Phụ lục 2: Hình ảnh tiêu bản của mẫu Tam thất




