19. Rao, K.S., et al., A novel method for synthesis of silica nanoparticles. Journal of colloid and interface science, 2005. 289(1): p. 125-131.
20. Jal, P., et al., Synthesis and characterization of nanosilica prepared by precipitation method. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004. 240(1-3): p. 173-178.
21. Silva, G.A., Introduction to nanotechnology and its applications to medicine.
Surgical neurology, 2004. 61(3): p. 216-220.
22. Shi, J., et al., Schiff based injectable hydrogel for in situ pH-triggered delivery of doxorubicin for breast tumor treatment. Polymer Chemistry, 2014. 5(21): p. 6180- 6189.
23. Karnati, S.R., et al., Application of surface-modified silica nanoparticles with dual silane coupling agents in bitumen for performance enhancement. Construction and Building Materials, 2020. 244: p. 118324.
24. Edrissi, M., M. Soleymani, and M. Adinehnia, Synthesis of Silica Nanoparticles by Ultrasound‐Assisted Sol‐Gel Method: Optimized by Taguchi Robust Design. Chemical engineering & technology, 2011. 34(11): p. 1813-1819.
25. Zohreh, N., S.H. Hosseini, and A. Pourjavadi, Hydrazine-modified starch coated magnetic nanoparticles as an effective pH-responsive nanocarrier for doxorubicin delivery. Journal of Industrial and Engineering Chemistry, 2016. 39: p. 203-209.
26. Sonawane, S.J., R.S. Kalhapure, and T. Govender, Hydrazone linkages in pH responsive drug delivery systems. European Journal of Pharmaceutical Sciences, 2017. 99: p. 45-65.
27. Narayan, R., et al., Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics, 2018. 10(3): p. 118.
28. Tomalia, D.A., Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Progress in Polymer Science, 2005. 30(3-4): p. 294-324.
29. Garg, U., et al., Current advances in chitosan nanoparticles based drug delivery and targeting. Advanced pharmaceutical bulletin, 2019. 9(2): p. 195.
30. Bernkop-Schnürch, A. and S. Dünnhaupt, Chitosan-based drug delivery systems.
European journal of pharmaceutics and biopharmaceutics, 2012. 81(3): p. 463-469.
31. Wübbeler, J.H., et al., Biodegradation of the xenobiotic organic disulphide 4, 4′- dithiodibutyric acid by Rhodococcus erythropolis strain MI2 and comparison with the microbial utilization of 3, 3′-dithiodipropionic acid and 3, 3′-thiodipropionic acid. Microbiology, 2010. 156(4): p. 1221-1233.
32. Qiu, L., C.-Y. Hong, and C.-Y. Pan, Doxorubicin-loaded aromatic imine-contained amphiphilic branched star polymer micelles: synthesis, self-assembly, and drug delivery. International journal of nanomedicine, 2015. 10: p. 3623.
33. Wang, Y., et al., A charge-conversional intracellular-activated polymeric prodrug for tumor therapy. Polymer Chemistry, 2016. 7(12): p. 2253-2263.
34. Ding, Y., et al., Polymerizable disulfide paclitaxel prodrug for controlled drug delivery. Materials Science and Engineering: C, 2014. 44: p. 386-390.
35. Cuong, N.-V., Y.-L. Li, and M.-F. Hsieh, Targeted delivery of doxorubicin to human breast cancers by folate-decorated star-shaped PEG–PCL micelle. Journal of Materials Chemistry, 2012. 22(3): p. 1006-1020.
104
36. Li, H., et al., Reduction-responsive drug delivery based on mesoporous silica nanoparticle core with crosslinked poly (acrylic acid) shell. Materials Science and Engineering: C, 2013. 33(6): p. 3426-3431.
37. Su, K. and C. Wang, Recent advances in the use of gelatin in biomedical research.
Biotechnology letters, 2015. 37(11): p. 2139-2145.
38. Tran, S., et al., Cancer nanomedicine: a review of recent success in drug delivery.
Clinical and translational medicine, 2017. 6(1): p. 44.
39. Longley, D.B., D.P. Harkin, and P.G. Johnston, 5-fluorouracil: mechanisms of action and clinical strategies. Nature reviews cancer, 2003. 3(5): p. 330-338.
40. Lung, N.T., Vai trò xét nghiệm đa hình gen đối với chỉnh liều 5-Fluorouracil (5-FU) trong điều trị ung thư. 2017.
41. Khoa, N.C., Dendrimer: Tổng hợp và ứng dụng trong y-dược. Nhà xuất bản Khoa học tự nhiên và công nghệ Hà Nội, , 2015.
42. Sweetman, S.C., Martindale: the complete drug reference. Vol. 3709. 2009: Pharmaceutical press London.
43. Yeager, C.E. and E.A. Olsen, Treatment of chemotherapy‐induced alopecia.
Dermatologic therapy, 2011. 24(4): p. 432-442.
44. Khoa, N.C., Vật liệu polyme thông minh và ứng dụng trong y khoa. Nhà xuất bản Khoa học tự nhiên và công nghệ Hà Nội, 2016. 64: p. 450-455.
45. Lâm, T.Đ., Vật liệu Nano sinh học. Nhà xuất bản Khoa học tự nhiên và công nghệ Hà Nội, 1993: p. 43-47.
46. van der Meel, R., Targeted inhibition of tumor growth and angiogenesis. 2013, University Utrecht.
47. Vallet-Regí, M., et al., Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules, 2018. 23(1): p. 47.
48. Vallet-Regi, M., et al., A new property of MCM-41: drug delivery system. Chemistry of Materials, 2001. 13(2): p. 308-311.
49. HuhandY, A. and J. Kwon, Nanoantibiotics’: anewparadigmfortreatinginfectiousdis‐ easesusingnanomaterialsintheantibioticsresistantera. JournalofControlledRelease, 2011. 156: p. 128-145.
50. Dvir, T., et al., Nanowired three-dimensional cardiac patches. Nature nanotechnology, 2011. 6(11): p. 720-725.
51. Tang, H., et al., Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery. International journal of pharmaceutics, 2011. 421(2): p. 388-396.
52. Nguyen, M.-N.T. and T.-D. Ho-Huynh, Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia long, against MCF-7 cells by synergistic effects. BMC complementary and alternative medicine, 2016. 16(1): p. 1-10.
53. Vien, T.A., et al., Antifungal, antibacterial and cytotoxic activities of some Ardisia species from Vietnam. Academia Journal of Biology, 2016. 38(1): p. 75-80.
54. Rahman, I.A. and V. Padavettan, Synthesis of silica nanoparticles by sol-gel: size- dependent properties, surface modification, and applications in silica-polymer nanocomposites—a review. Journal of Nanomaterials, 2012. 2012.
55. Tuấn, N.T., et al., Tổng hợp hạt nano SiO2 từ tro vỏ trấu bằng phương pháp kết tủa.
Tạp chí Khoa học Trường Đại học Cần Thơ, 2014. 32: p. 120-124.
105
56. Sun, L., et al., Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian journal of pharmaceutical sciences, 2017. 12(5): p. 418-423.
57. Wang, X., et al., Increasing the cytotoxicity of doxorubicin in breast cancer MCF-7 cells with multidrug resistance using a mesoporous silica nanoparticle drug delivery system. International journal of clinical and experimental pathology, 2014. 7(4): p. 1337.
58. Cuong, N.-V., et al., Doxorubicin-loaded PEG-PCL-PEG micelle using xenograft model of nude mice: Effect of multiple administration of micelle on the suppression of human breast cancer. Cancers, 2010. 3(1): p. 61-78.
59. Danaei, M., et al., Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics, 2018. 10(2): p. 57.
60. Bogush, G. and C. Zukoski Iv, Studies of the kinetics of the precipitation of uniform silica particles through the hydrolysis and condensation of silicon alkoxides. Journal of Colloid and Interface Science, 1991. 142(1): p. 1-18.
61. IA Rahman, V.P., Synthesis of silica nanoparticles by sol-gel: size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—a review. Journal of Nanomaterials, 2012: p. 2-3.
62. Stöber, W., A. Fink, and E. Bohn, Controlled growth of monodisperse silica spheres in the micron size range. Journal of colloid and interface science, 1968. 26(1): p. 62- 69.
63. Bogush, G., M. Tracy, and C. Zukoski Iv, Preparation of monodisperse silica particles: control of size and mass fraction. Journal of non-crystalline solids, 1988. 104(1): p. 95-106.
64. Van Helden, A., J. Jansen, and A. Vrij, Preparation and characterization of spherical monodisperse silica dispersions in nonaqueous solvents. Journal of colloid and interface science, 1981. 81(2): p. 354-368.
65. Matsoukas, T. and E. Gulari, Dynamics of growth of silica particles from ammonia- catalyzed hydrolysis of tetra-ethyl-orthosilicate. Journal of colloid and interface science, 1988. 124(1): p. 252-261.
66. Biricik, H. and N. Sarier, Comparative study of the characteristics of nano silica-, silica fume-and fly ash-incorporated cement mortars. Materials Research, 2014. 17(3): p. 570-582.
67. Liou, T.-H. and C.-C. Yang, Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Materials science and engineering: B, 2011. 176(7): p. 521-529.
68. Lehman, S.E., Spectroscopic studies of silica nanoparticles: magnetic resonance and nanomaterial-biological interactions. 2016.
69. Sun, J., et al., Effect of nano-SiO2 on the early hydration of alite-sulphoaluminate cement. Nanomaterials, 2017. 7(5): p. 102.
70. Ghosh, R. and S. Bhattacherjee, A review study on precipitated silica and activated carbon from rice husk. J Chem Eng Process Technol, 2013. 4(4): p. 1-7.
71. Rafi, A.A., et al., A Smart pH-responsive Nano-Carrier as a Drug Delivery System: A hybrid system comprised of mesoporous nanosilica MCM-41 (as a nano-container) & a pH-sensitive polymer (as smart reversible gatekeepers): Preparation, characterization and in vitro release studies of an anti-cancer drug. European Journal of Pharmaceutical Sciences, 2016. 93: p. 64-73.
106
72. Phương, H.T. and N.K.D. Hồng, Study on the surface functionalization of nanosilica for oil adsorption. Vietnam Journal of Science and Technology, 2016. 54(6): p. 755.
73. Stojanovic, D., et al., Preparation of MEMO silane-coated SiO2 nanoparticles under high pressure of carbon dioxide and ethanol. The Journal of Supercritical Fluids, 2010. 52(3): p. 276-284.
74. Kalapathy, U., A. Proctor, and J. Shultz, A simple method for production of pure silica from rice hull ash. Bioresource technology, 2000. 73(3): p. 257-262.
75. Jafari, V., A. Allahverdi, and M. Vafaei, Ultrasound-assisted synthesis of colloidal nanosilica from silica fume: Effect of sonication time on the properties of product. Advanced Powder Technology, 2014. 25(5): p. 1571-1577.
76. Jafari, V. and A. Allahverdi, Synthesis of nanosilica from silica fume using an acid- base precipitation technique and PVA as a nonionic surfactant. Journal of Ultrafine Grained and Nanostructured Materials, 2014. 47(2): p. 105-112.
77. González, M.G., J.C. Cabanelas, and J. Baselga, Applications of FTIR on epoxy resins-identification, monitoring the curing process, phase separation and water uptake. Infrared Spectroscopy-Materials Science, Engineering and Technology, 2012. 2: p. 261-284.
78. Meza-Arroyo, J., et al., Low temperature processing of Al2O3-GPTMS-PMMA hybrid films with applications to high-performance ZnO thin-film transistors. Applied Surface Science, 2019. 467: p. 456-461.
79. Rafigh, S.M. and A. Heydarinasab, Mesoporous chitosan–SiO2 nanoparticles: synthesis, characterization, and CO2 adsorption capacity. ACS Sustainable Chemistry & Engineering, 2017. 5(11): p. 10379-10386.
80. Liu, Y., et al., A self-monitored fluorescence DNA anti-counterfeiting system based on silica coated SYBR Green I/DNA gelatin nanoparticles. Journal of Materials Chemistry C, 2017. 5(24): p. 5939-5948.
81. Zhang, J., et al., Mesoporous silica nanoparticles with redox-responsive surface linkers for charge-reversible loading and release of short oligonucleotides. Dalton Transactions, 2014. 43(10): p. 4115-4126.
82. Feng, X., et al., Schiff base bond-linked polysaccharide–doxorubicin conjugate for upregulated cancer therapy. Materials Science and Engineering: C, 2017. 76: p. 1121-1128.
83. Sharma, M.V.P., et al., An efficient and novel porous nanosilica supported TiO2 photocatalyst for pesticide degradation using solar light. Journal of Hazardous Materials, 2009. 171(1-3): p. 626-633.
84. Ek, S., et al., Determination of the hydroxyl group content in silica by thermogravimetry and a comparison with 1H MAS NMR results. Thermochimica acta, 2001. 379(1-2): p. 201-212.
85. She, X., et al., Functionalization of hollow mesoporous silica nanoparticles for improved 5-FU loading. Journal of Nanomaterials, 2015. 2015.
86. Kamba, S.A., et al., In vitro delivery and controlled release of doxorubicin for targeting osteosarcoma bone cancer. Molecules, 2013. 18(9): p. 10580-10598.
87. Manocha, B. and A. Margaritis, Controlled release of doxorubicin from doxorubicin/-polyglutamic acid ionic complex. Journal of Nanomaterials, 2010. 2010.
88. Llinas, M.C., et al., Preparation of a mesoporous silica-based nano-vehicle for dual DOX/CPT pH-triggered delivery. Drug delivery, 2018. 25(1): p. 1137-1146.
89. Patil, R., et al., Cellular delivery of doxorubicin via pH-controlled hydrazone linkage using multifunctional nano vehicle based on poly (β-L-malic acid). International journal of molecular sciences, 2012. 13(9): p. 11681-11693.
90. Ghosh, S., S.K. Goswami, and L.J. Mathias, Surface modification of nano-silica with amides and imides for use in polyester nanocomposites. Journal of Materials Chemistry A, 2013. 1(19): p. 6073-6080.
91. Tao, Y., et al., A pH-responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior. Polymer Chemistry, 2018. 9(7): p. 878-884.
92. Najafi, F., et al., Effect of grafting ratio of poly (propylene imine) dendrimer onto gold nanoparticles on the properties of colloidal hybrids, their DOX loading and release behavior and cytotoxicity. Colloids and Surfaces B: Biointerfaces, 2019. 178: p. 500-507.
93. Qiao, Z.-Y., et al., Multi-responsive nanogels containing motifs of ortho ester, oligo (ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. Journal of controlled release, 2011. 152(1): p. 57-66.
94. Kuang, G., et al., Reduction-responsive disulfide linkage core-cross-linked polymeric micelles for site-specific drug delivery. Polymer Chemistry, 2020. 11(44): p. 7078- 7086.
95. Zhai, Y., et al., Design, synthesis, and characterization of Schiff base bond-linked pH-responsive doxorubicin prodrug based on functionalized mPEG-PCL for targeted cancer therapy. Polymers, 2018. 10(10): p. 1127.
96. DiazDuarte-Rodriguez, M., et al., Dual responsive polymersomes for gold nanorod and doxorubicin encapsulation: nanomaterials with potential use as smart drug delivery systems. Polymers, 2019. 11(6): p. 939.
97. Sun, Y., et al., RGD Peptide‐Based Target Drug Delivery of Doxorubicin Nanomedicine. Drug development research, 2017. 78(6): p. 283-291.
98. Wahbeh, J. and S. Milkowski, The use of hydrazones for biomedical applications. SLAS TECHNOLOGY: Translating Life Sciences Innovation, 2019. 24(2): p. 161- 168.
99. Qu, X. and Z. Yang, Benzoic‐Imine‐Based Physiological‐pH‐Responsive Materials for Biomedical Applications. Chemistry–An Asian Journal, 2016. 11(19): p. 2633- 2641.
100. Hira, S.K., et al., Targeted delivery of doxorubicin-loaded poly (ε-caprolactone)-b- poly (N-vinylpyrrolidone) micelles enhances antitumor effect in lymphoma. Plos one, 2014. 9(4): p. e94309.
101. Nguyen-Thi, N.-T., et al., The Engineering of Porous Silica and Hollow Silica Nanoparticles to Enhance Drug-loading Capacity. Processes, 2019. 7(11): p. 805.
PHỤ LỤC
Phụ lục 1. Bảng khảo sát các yếu tố ảnh hưởng đến kích thước hạt nano silica.
Khảo sát ảnh hưởng NH3 (%) | Khảo sát ảnh hưởng ethanol (mL) | Kích thước hạt nano silica (nm) | |
6 | 2,8% | 11,25 | 92,8 ± 10.8 |
8 | 2,8% | 11,25 | 60,0 ± 19.8 |
10 | 2,8% | 11,25 | 56,6 ± 8.3 |
12 | 2,8% | 11,25 | 109,6 ± 19.3 |
14 | 2,8% | 11,25 | 153,8 ± 25.1 |
8 | 14,0% | 11,25 | 79,0 ± 15.1 |
8 | 28,0% | 11,25 | 136,0 ± 27.1 |
8 | 2,8% | 5,80 | 57,0 ± 13.1 |
8 | 2,8% | 17,50 | 153,0 ± 25.3 |
8 | 2,8% | 23,30 | 192,0 ± 11.1 |
8 | 2,8% | 29,10 | 212,0 ± 10.1 |
Có thể bạn quan tâm!
-
 Phổ Hplc Của 5-Fu (A) Và Hplc Của Hệ Pns-Gptms-Cs-Mpeg Mang 5-Fu Giải Phóng (B)
Phổ Hplc Của 5-Fu (A) Và Hplc Của Hệ Pns-Gptms-Cs-Mpeg Mang 5-Fu Giải Phóng (B) -
 Phổ Hplc Của 5-Fu (A) Và Hplc Của Hệ Pns-Aptes-Cooh-Ge
Phổ Hplc Của 5-Fu (A) Và Hplc Của Hệ Pns-Aptes-Cooh-Ge -
 Nhận Xét Chung Cho Tất Cả Các Kết Quả Mang Và Giải Phóng Thuốc
Nhận Xét Chung Cho Tất Cả Các Kết Quả Mang Và Giải Phóng Thuốc -
 Nghiên cứu biến tính bề mặt nano silica làm chất mang thuốc chống ung thư - 17
Nghiên cứu biến tính bề mặt nano silica làm chất mang thuốc chống ung thư - 17
Xem toàn bộ 137 trang tài liệu này.
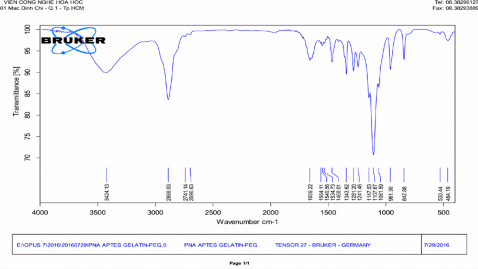
Phụ lục 2. Kết quả FTIR của PNS-GPTMS
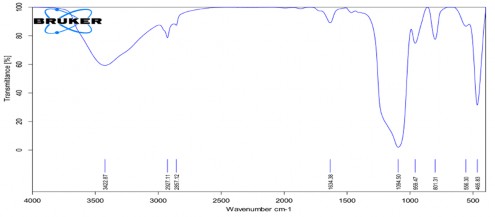
Phụ lục 3. Kết quả FTIR của CS-mPEG
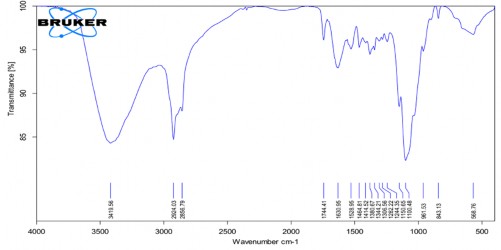
Phụ lục 4. Kết quả FTIR của PNS-GPTMS-CS-mPEG
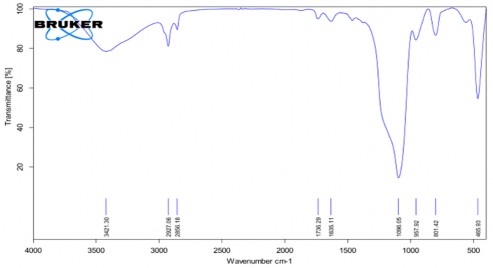
Phụ lục 5. Kết quả FTIR của PNS-APTES
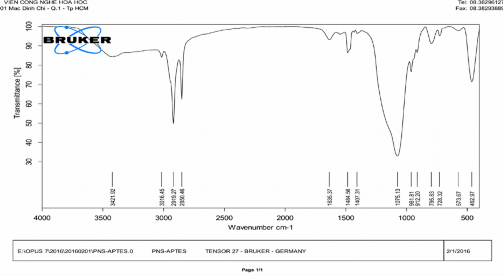
Phụ lục 6. Kết quả FTIR của Gelatin-mPEG
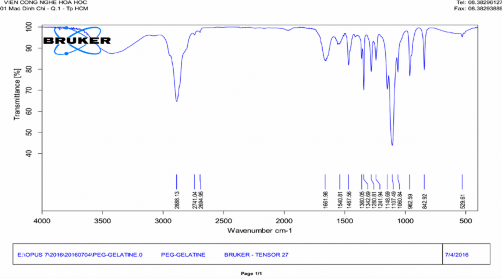
Phụ lục 7. Kết quả FTIR của PNS-APTES-COOH-Gelatin-mPEG